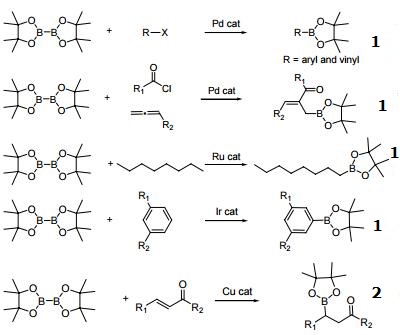
B2pin2-mediated copper-catalyzed oxidation of alkynes into 1,2-diketones using molecular oxygen - ScienceDirect

Understanding the Higher Reactivity of B2cat2 versus B2pin2 in Copper(I)-Catalyzed Alkene Diboration Reactions | Organometallics

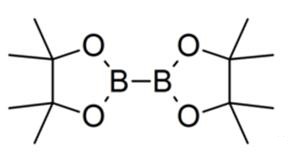
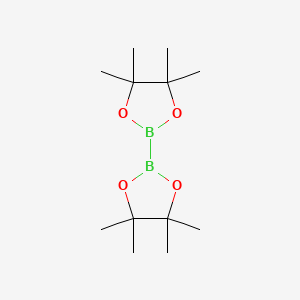
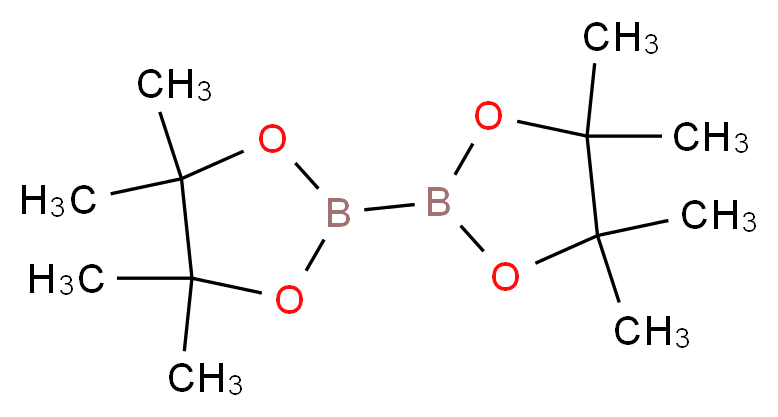
73183-34-3 | Bis(pinacolato)diboron | 2-(4,4,5,5-Tetramethyl-1,3,2 -dioxaborolan-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; 4,4,4',4',5,5,5',5'-Octamethyl-2,2'-bis(1,3,2-dioxaborolane); 4,4',5,5'-Octamethyl-2,2'-bi-1,3,2-dioxaborolane; B2Pin2; Bis ...

B2pin2-mediated copper-catalyzed oxidation of alkynes into 1,2-diketones using molecular oxygen - ScienceDirect

Base-Catalyzed Borylation/B–O Elimination of Propynols and B2pin2 Delivering Tetrasubstituted Alkenylboronates | Organic Letters

Nanomaterials | Free Full-Text | α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base

Enantioselective Cu(I)-catalyzed borylative cyclization of enone-tethered cyclohexadienones and mechanistic insights | Nature Communications
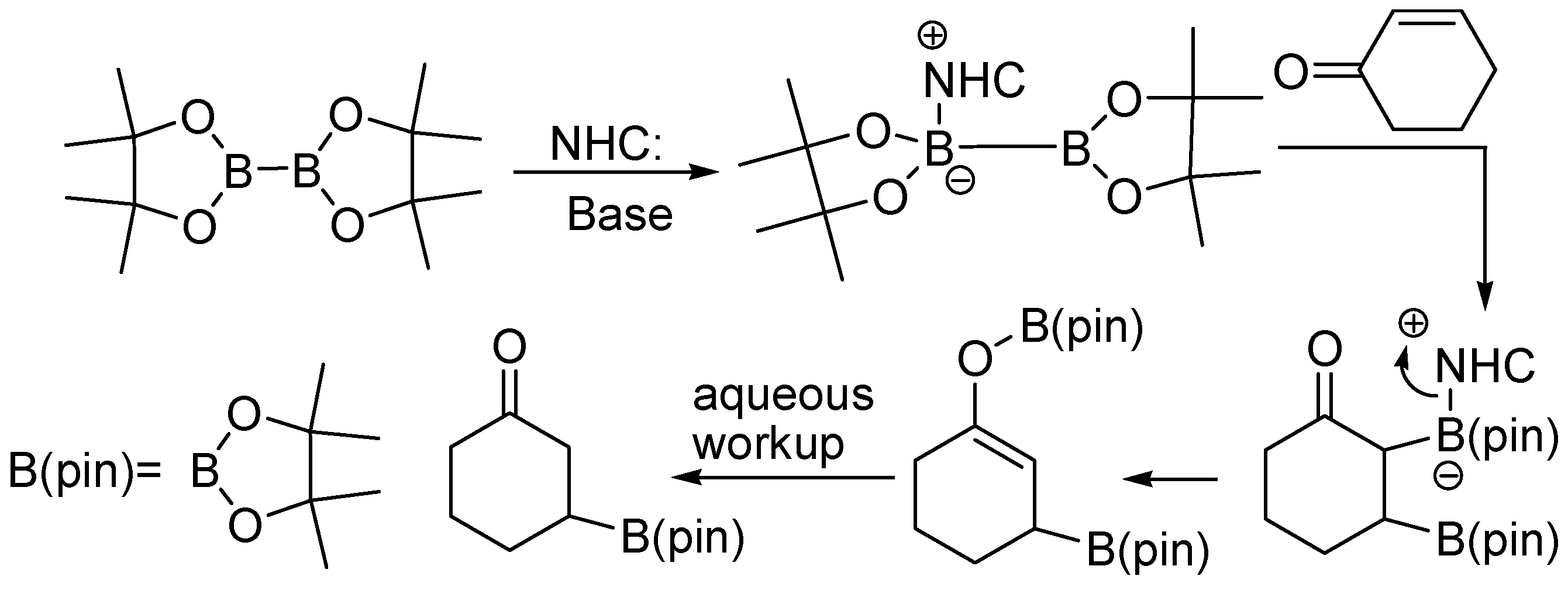
Molecules | Free Full-Text | Recent Synthesis Developments of Organoboron Compounds via Metal-Free Catalytic Borylation of Alkynes and Alkenes
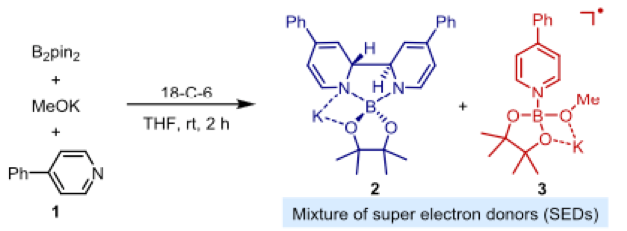
Boron sees the light- A visible light induced organocatalytic borylation of aryl chlorides - Scientific Update - UK

Copper-catalyzed borofunctionalization of styrenes with B2pin2 and CO† † Electronic supplementary information (ESI) available: General comments, general procedure, analytical data, and NMR spectra. See DOI: 10.1039/d1sc04774d - Abstract - Europe PMC

B–B bond activation and NHC ring-expansion reactions of diboron(4) compounds, and accurate molecular structures of B2(NMe2)4, B2eg2, B2neop2 and B2pin2 - Dalton Transactions (RSC Publishing)